
Common Conditions After Surgery
If you have been diagnosed with any of these medical conditions after getting a Cartiva Synthetic Cartilage Implant (Cartiva SCI), you may be entitled to compensation:
- Joint irritation
- Fibrosis
- Joint instability, joint malalignment
- Revision or conversion to fusion
- Allergic reaction to polyvinyl alcohol (PVA)
- Progressive osteoarthritis (OA)
- Incorrect implant placement
- Damage to adjacent or surrounding tissues
- Periarticular cyst
- Bone cyst or bone loss
- Sesamoid bone(s) irritation or fracture
- Implant fracture, loosening, dislocation, dislodgment, or subsidence
- Infection
- Inflammation, pain, or swelling
- Effusion
- Metatarsal bone fracture
- Osteonecrosis
- Avascular necrosis
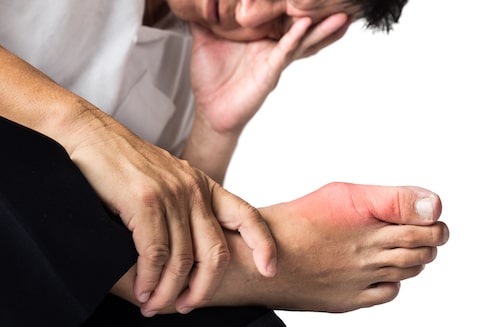
While some patients are satisfied with Cartiva®, the majority of those who received the Cartiva® are dissatisfied with the procedure and have various complaints, including that the pain lasts longer than expected.
Click Here To Lean MoreWarning: Failure to seek and receive effective treatment for arthritis in the big toe can have a significant impact on the patient’s gait, which results in chronic hip and/or knee pain, among other things.
What is the Cartiva SCI?
The FDA approved the Cartiva Synthetic Cartilage Implant in mid-2016 to treat a painful degenerative or post-traumatic condition - mid-stage to advanced arthritis occurring at the base of the big toe.
Cartiva® is an implant made of saline solution and polyvinyl alcohol hydrogel, a material similar to what is used in contact lenses. The implant itself is approximately the shape and size of a jelly bean and it acts as a replacement for cartilage, providing cushioning to the bones in the toe joint allowing patients to maintain a full range of motion in their toe.
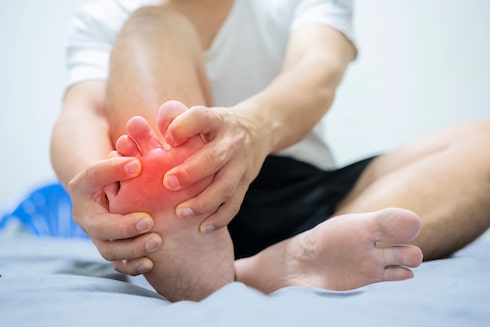
The Cartiva® implant surgery takes somewhere between 30 and 45 minutes during which time the surgeon makes a small incision over the toe joint, removes damaged cartilage, and inserts the Cartiva® implant to replace the cartilage. A patient receiving a Cartiva® implant should be able to walk immediately after surgery.
Those individuals who suffer from arthritis of the big toe understand the impact such a condition can have, and how dramatically it curtails one's ability to remain active.
Because the big toe absorbs most of the pressure from walking, those suffering from this type of arthritis often have trouble jogging, let alone walking. Because this condition is degenerative, as the cartilage protecting the big toe joint wears away over time, the bones in the main joint of the big toe (metatarsophalangeal) rub against each other, and cause chronic pain, swelling, and stiffness.
Get Your Free Claim Review Today
You Are Not Alone - We Are Here To Help
We believe that patients who were diagnosed with a serious medical condition after surgery to insert a Cartiva® synthetic cartilage implant should be able to hold the manufacturer responsible to the full extent of the law.
Get Your Free Review